What are Isotopes?
Let’s believe a couple of equal twins. These twins have the identical temperament, and due to the fact they are equal, it’s miles very tough to inform them aside until you look at them closely. When it’s time for his or her annual physical, the twins want to step on a weighing scale, and after they do, one weighs barely extra than the different. In phrases of chemistry, we will say that those twins are like isotopes of every different.
Atoms and factors are product of protons, neutrons and electrons. The nucleus is product of protons and neutrons, and the electrons surround the nucleus, as proven within side the instance under. The sum of the quantity of protons and the quantity of neutrons is identical to the atomic mass.
In a given detail, the quantity of neutrons may be extraordinary from every different, even as the quantity of protons is now no longer. These extraordinary variations of the identical detail are known as isotopes. Isotopes are atoms with the identical quantity of protons however which have a extraordinary quantity of neutrons. Since the atomic quantity is identical to the quantity of protons and the atomic mass is the sum of protons and neutrons, we also can say that isotopes are factors with the identical atomic quantity however extraordinary mass numbers.
Let us test an example.
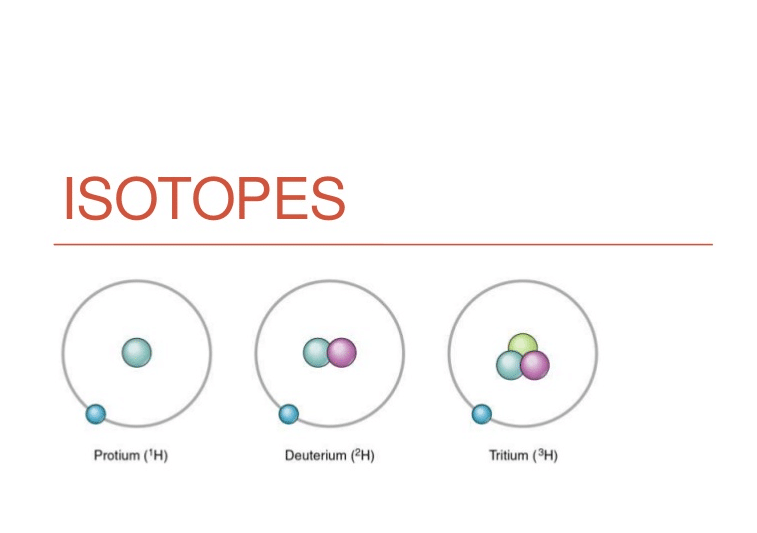
Isotopes of Hydrogen
The 3 are all isotopes of hydrogen. As you could see, they have got the identical atomic quantity, or quantity of protons, (quantity on the decrease left of the detail) however extraordinary atomic loads (quantity on the top left of the detail).
The quantity of neutrons may be calculated via way of means of calculating the distinction among the atomic mass and atomic quantity. We can see that for the isotopes of hydrogen, they have got various quantity of neutrons. For protium, the quantity of neutrons is zero; for deuterium, the quantity of neutrons is one; and for tritium, the quantity of neutrons is .
Going returned to our assessment with equal twins, we will say that those 3 isotopes of hydrogen are like equal triplets of every different – they will look like equal outside, however they’re extraordinary inside, and in addition they have extraordinary names.
Isotopes of Carbon
A very famous detail, carbon, additionally has isotopes. There are 3 isotopes of carbon: carbon-12, carbon-thirteen and carbon-14. The numbers which might be after the carbon confer with the atomic mass.
The maximum not unusual place and considerable isotope of carbon is carbon-12. Looking at the chances under every carbon isotope, we see that nearly 98.9% of the carbon this is determined is within side the shape of carbon-12. The least considerable shape of carbon is carbon-14, with an abundance of much less than 0.0001%. If we calculate the quantity of neutrons for every carbon isotope, we will see that they fluctuate from every different. For carbon-12, we’ve 6 neutrons; for carbon-thirteen, we’ve 7 neutrons; and for carbon-14, we’ve eight neutrons.
You might also additionally word if we examine the atomic loads of factors. Within side the periodic desk that they’re hardly ever ever complete numbers. Moreover, similar to for carbon wherein the atomic mass is 12.011. This is due to the fact the atomic mass of carbon is primarily based at the common atomic loads of its isotopes. And the abundance of every isotope.
Types of Isotopes
There are fundamental varieties of isotopes. Those are radioactive isotopes and solid isotopes. Stable isotopes have a solid aggregate of protons and neutrons, so that they have solid nuclei and do now no longer go through decay. These isotopes do now no longer pose risky results to residing things, like radioactive isotopes.
They are commonly beneficial while appearing experiments within side the surroundings and within side the area of geochemistry. Additionally these isotopes can assist decide the chemical composition and age of minerals and different geologic objects. Some examples of solid isotopes are isotopes of carbon, potassium, calcium and vanadium.