How to Calculate Specific Heat
Have you ever puzzled why it takes goodbye to boil water? Do you recognize that it takes longer to boil a few beverages than it does others? For instance, in case you have been to warmness the identical quantity of water and alcohol, the alcohol boils first. Well, what debts for this distinction? The solution is precise warmness ability.
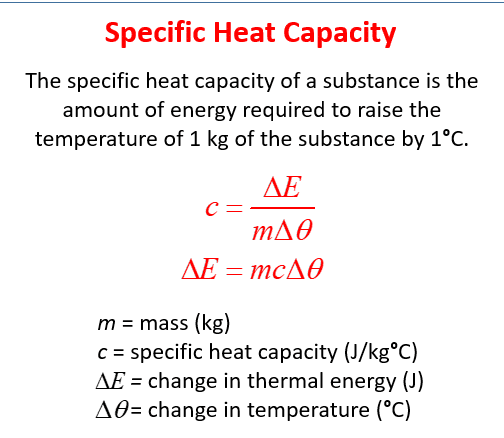
Specific warmness ability, or simply precise warmness, is the quantity of warmth required to alternate the temperature of a substance. As water calls for greater time to boil than does alcohol, you may finish that water calls for greater warmness than alcohol to elevate its temperature. In different words, water has a better precise warmness ability. That end could be accurate. To higher recognize precise warmness ability, we want to differentiate among warmness and temperature, as each of those phrases are utilized in its definition.
The Difference Between Heat and Temperature
Let’s have a take a observe an instance to assist us recognize the distinction among warmness and temperature. Consider beakers of boiling water with 1 liter in a single and a pair of liters within side the different. If each are boiling, they have got the identical temperature. You see, temperature is described because the common quantity of kinetic strength within side the substance, where kinetic strength is the strength of movement. The molecules in every pot have the identical common strength of movement or common quantity of kinetic strength. In different words, the water molecules in every pot are transferring on the identical speed. Temperature is measured in levels Celsius, levels Fahrenheit, or maybe Kelvin.
However, liters of boiling water include greater warmness than one liter of boiling water, despite the fact that they have got the identical temperature. In the context of precise warmness ability. Warmness is the full quantity of strength in a substance, and warmth is on occasion refer warmness strength. In which strength is the cappotential to do work. In the clinical community, strength is measure in joules however additionally may express as calories.
While warmness and temperature are exclusive measures, they’re in reality related. Temperature will increase as warmness is deliver to a substance. Likewise, temperature decreases as warmness is remove.
How to Calculate Specific Heat Capacity
Let’s do some experiment. All we want is a beaker of water, a thermometer, and a strength deliver that generates warmness. The strength deliver will degree the quantity of warmth that we upload to the water. Let’s say that we warmness 10 mL (10 grams) of water 10 levels Celsius. In doing so, the strength deliver registers 420 joules. That method it took 420 joules of warmth strength to elevate 10 grams of water 10 levels Celsius.
We can use this data to calculate the precise warmness ability of water. All we want is to discern out how a lot warmness to elevate the temperature of 1 gram of water one diploma Celsius.
Specific Heat Capacity: This lesson relates warmness to a alternate in temperature. We speak how the quantity of warmth wanted for a temperature alternate is depending on mass and the substance involve, and that courting is represent with the aid of using the precise warmness capacity of the substance, C.
The dependence on temperature alternate and mass are without problems refer. Because the (common) kinetic strength of an atom or molecule is proportional to absolutely the temperature, the inner strength of a gadget is proportional to absolutely the temperature and the variety of atoms or molecules. Since the transferred warmness is same to the alternate within side the inner strength, the warmth is proportional to the mass of the substance and the temperature alternate. The transferred warmness additionally relies upon at the substance so that, for instance, the warmth important to elevate the temperature is much less for alcohol than for water. For the identical substance, the transferred warmness additionally relies upon at the phase (gas, liquid, or solid).